Make the Change for Kidney Health

Chronic kidney disease – CKD – poses a growing burden across the globe. CKD affects more than 10% of the world’s population, or approximately 850 million people, and is projected to be the world’s fifth leading cause of death by 2040. Yet it’s not considered a priority among non-communicable diseases.
Kidney Health for All

Chronic kidney disease affects more than 10% of the world’s population, around 850 million people. World Kidney Day aims to bring that number down – by raising awareness, advancing screening and improving access to treatment.
Illuminating Advocacy: Rare Disease Day

February 29 unites more than 300 million people worldwide who share a common challenge: living with a rare disease.
New Global Patient Alliance Is Raising Profile of Chronic Kidney Disease

A new global coalition, the Global Patient Alliance for Kidney Health, launched last week. With 17 member organizations spanning five continents, the Alliance hopes to improve screening access and early treatment for a serious but often neglected public health challenge: chronic kidney disease, or CKD.
New Kidney Health Alliance Elevates Chronic Kidney Disease on Global Health Agenda

Seventeen patient advocacy organizations from North and South America, Europe, Asia and the Middle East have formed the Global Patient Alliance for Kidney Health. The Alliance aims to elevate patient voices and advocate for policies that enhance access to screening and early treatment of chronic kidney disease, or CKD.
Calculating Cardiovascular Risk in Australia

One in every four deaths in Australia stems from cardiovascular disease, according to 2021 data. And more than 1 million Australians live with heart disease, stroke or vascular conditions.
Know Your Numbers, Treat Your Risk
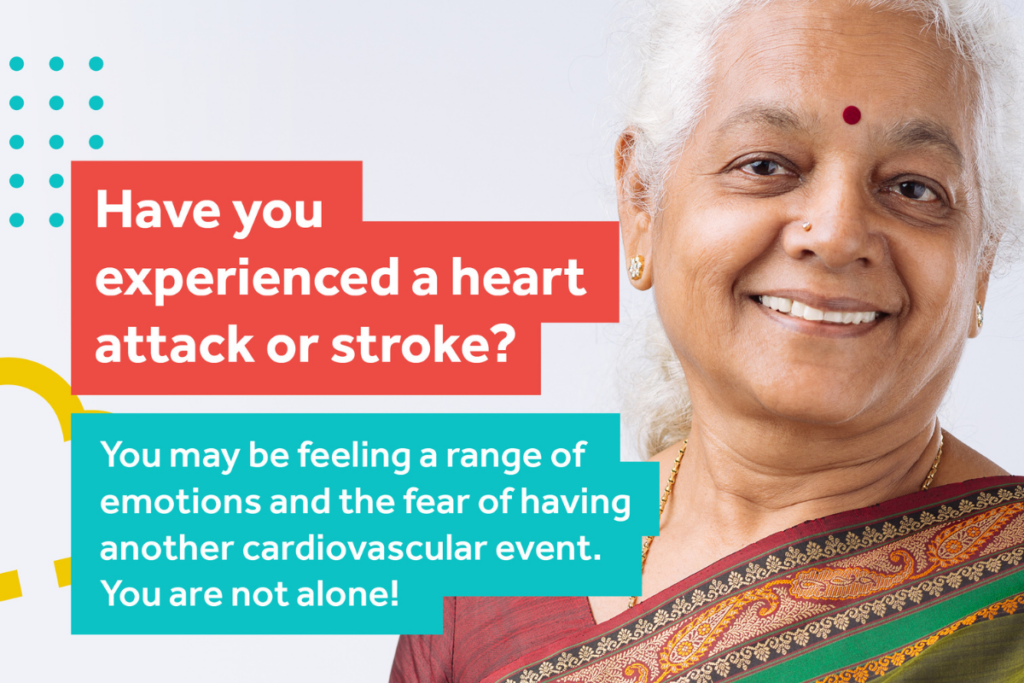
Every 10 minutes someone in Australia has a heart attack.
COVID-19 Vaccination in Developing Nations
This World Amyloidosis Day, Be the Link

Amyloidosis. For thousands of people around the world, this one word, this one diagnosis, can stop life in its tracks. But for many, it’s a word they’ve never heard before.
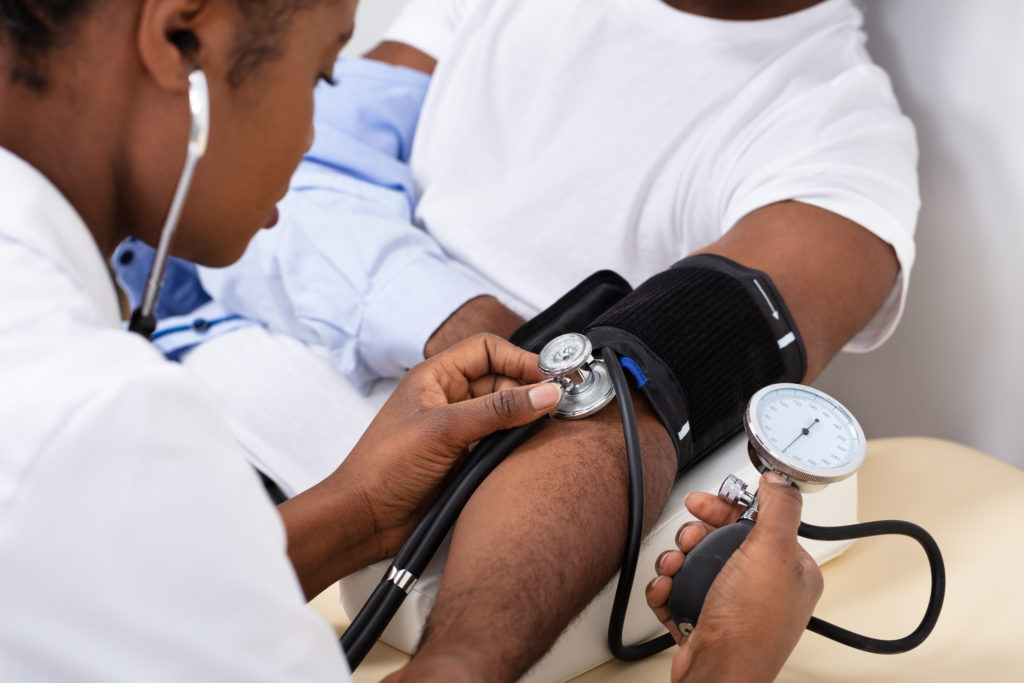
High Blood Pressure Tops 1 Billion People Across the Globe