Make the Change for Kidney Health

Chronic kidney disease – CKD – poses a growing burden across the globe. CKD affects more than 10% of the world’s population, or approximately 850 million people, and is projected to be the world’s fifth leading cause of death by 2040. Yet it’s not considered a priority among non-communicable diseases.
Kidney Health for All

Chronic kidney disease affects more than 10% of the world’s population, around 850 million people. World Kidney Day aims to bring that number down – by raising awareness, advancing screening and improving access to treatment.
Illuminating Advocacy: Rare Disease Day

February 29 unites more than 300 million people worldwide who share a common challenge: living with a rare disease.
Protecting Patient Access to Clinical Trials in Europe

An all-encompassing revision of pharmaceutical laws from the European Commission aims to make medications more available, accessible and affordable. But these revisions could have unintended consequences for patients’ access to clinical trials and new treatments.
New Global Patient Alliance Is Raising Profile of Chronic Kidney Disease

A new global coalition, the Global Patient Alliance for Kidney Health, launched last week. With 17 member organizations spanning five continents, the Alliance hopes to improve screening access and early treatment for a serious but often neglected public health challenge: chronic kidney disease, or CKD.
New Kidney Health Alliance Elevates Chronic Kidney Disease on Global Health Agenda

Seventeen patient advocacy organizations from North and South America, Europe, Asia and the Middle East have formed the Global Patient Alliance for Kidney Health. The Alliance aims to elevate patient voices and advocate for policies that enhance access to screening and early treatment of chronic kidney disease, or CKD.
Traitement de l’hypertension artérielle: les nouvelles directives européennes ouvrent la voie à un meilleur accès à la procédure

La European Alliance for Patient Access se félicite de l’ajout de la dénervation rénale à la liste des traitements approuvés pour l’hypertension artérielle
Neue europäische Hypertonie-Leitlinien ebnen den Weg für einen verbesserten Zugang zu Bluthochdruckverfahren

European Alliance for Patient Access begrüßt die Aufnahme der renalen Denervierung in die Liste der empfohlenen Behandlungen für arteriellen Bluthochdruck
Las nuevas directrices europeas sobre hipertensión allanan el camino para un mayor acceso al procedimiento de hipertensión arterial

La Alianza Europea para el Acceso de Pacientes recomienda que se agregue la denervación renal a la lista de tratamientos aprobados para la hipertensión arterial
New European Hypertension Guidelines Pave the Way for Greater Access to High Blood Pressure Procedure
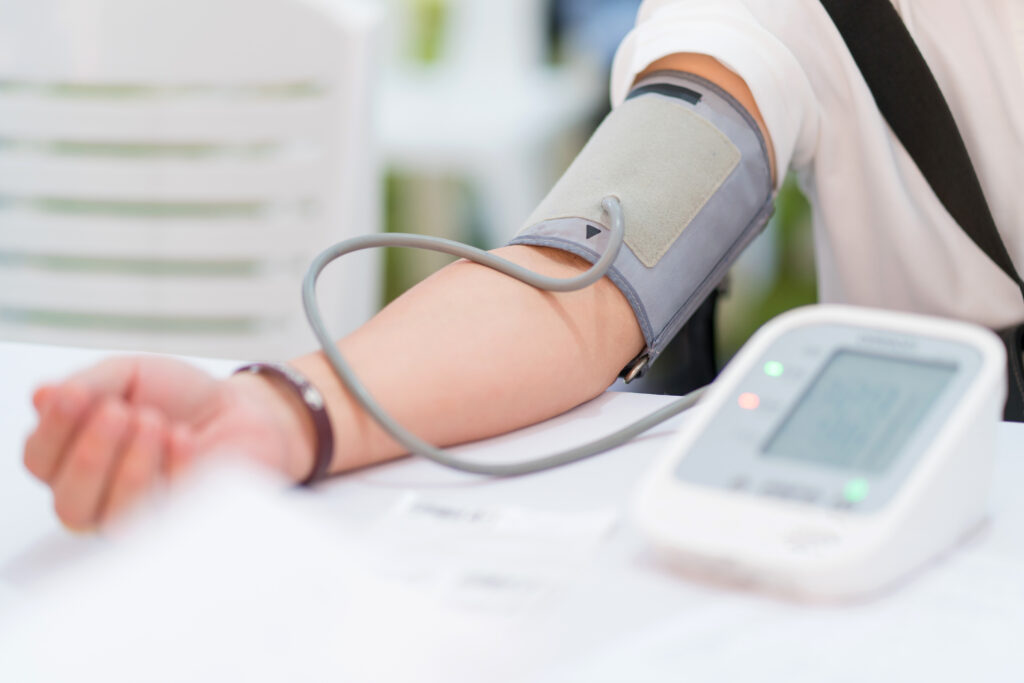
European Alliance for Patient Access commends renal denervation being added to list of endorsed treatments for arterial hypertension.

